
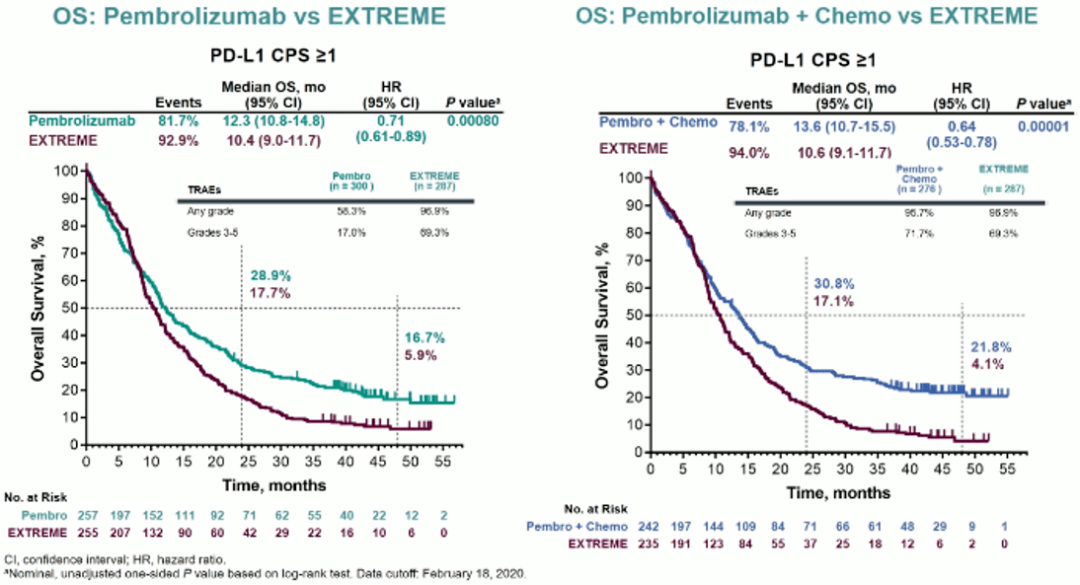
ResultsĨ04 pts were randomized (402 pts in each arm). OS and safety/tolerability were secondary endpoints. Primary endpoint was EFS (Efficacy boundary, one-sided P=0.0242). A pembro/placebo priming dose was given 1 wk before CRT, followed by 2 doses during CRT and 14 doses of maintenance therapy after CRT, for a total of 17 doses. Pts with newly diagnosed, pathologically proven, treatment-naive LA-HNSCC (T3–T4 or any N2a–3 larynx/hypopharynx/oral cavity/p16-negative oropharynx cancers and T4 or N3 p16-positive oropharynx cancer) who were eligible for definitive CRT were randomized (1:1) to pembro 200 mg Q3W + CRT (70Gy/35F + cisplatin 100 mg/m 2 Q3W) followed by pembro or placebo Q3W + CRT followed by placebo. The randomized, double-blind, phase 3 KEYNOTE-412 (NCT03040999) study investigated the efficacy and safety of pembro + CRT vs placebo + CRT in patients (pts) with LA-HNSCC. Pembro is approved as monotherapy or in combination with chemotherapy for recurrent/metastatic HNSCC. 20 Medical Oncology And Hematology Department, Princess Margaret Cancer Centre, M5G 1Z5 - Toronto/CA.19 Clinical, Merck & Co., Inc., Rahway/US.18 Oncology Clinical Research, Merck & Co., Inc., 07065 - Rahway/US.16 Clinical, Merck & Co., Inc., NJ 08889 - Rahway/US.15 Head And Neck Medical Oncology Unit, Fondazione IRCCS - Istituto Nazionale dei Tumori, 20133 - Milan/IT.14 Oncology, Andrzej Frycz Modrzewski Krakow University, Amethyst Radiotherapy Centre, Rydygier Hospital, Krakow/PL.13 Medical, Ordensklinikum Linz Barmherzige Schwestern, 4010 - Linz/AT.12 Medical, Paracelsus Medizinische Privatuniversität, 5020 - Salzburg/AT.11 Department Of Otorhinolaryngology And Head & Neck Surgery, University Hospital of Ulm - Ear, Nose and Throat Medicine, 89075 - Ulm/DE.10 Medical Oncology Department, Hacettepe University - Faculty of Medicine, 06100 - Ankara/TR.

9 Oncology, Centre Jean Bernard, 72000 - Le Mans/FR.8 Cancer Care Services, Royal Brisbane and Women's Hospital, 4029 - Herston/AU.7 Pesquisa Clínica, CRIO Centro Regional Integrado de Oncologia, 60335-480 - Fortaleza-CE/BR.6 Hemato-oncology Division, Hospital Nossa Senhora da Conceicao, 91350-200 - Porto Alegre/BR.5 Medical Oncology Department, Peter MacCallum Cancer Centre, 3000 - Melbourne/AU.4 Head And Neck Medical Oncology Dept., National Cancer Center Hospital East, 277-8577 - Kashiwa/JP.3 Internal Medicine Department, Yale University School of Medicine - Yale Cancer Center, 06520 - New Haven/US.2 Radiation Oncology, Institut Gustave Roussy, 94805 - Villejuif, Cedex/FR.1 Department Of Oncology, Cliniques Universitaires Saint-Luc (UCLouvain Saint-Luc) and Institut de Recherche Clinique et Expérimentale (Pole MIRO), 1200 - Woluwe-Saint-Lambert/BE.


 0 kommentar(er)
0 kommentar(er)
